Comprehensive consulting: starting from the selection of a suitable sterilization process to the design of a turnkey system

Globally, approx. 60% of medical devices are sterilized with ethylene oxide. For this sterilization method, two processes are distinguished: the vacuum sterilization process and the overpressure sterilization process.
Thus, before purchasing a new system, you must first choose the right process which corresponds to your production process. We will gladly help you choose by analyzing your underlying conditions, such as device and packaging characteristics and production volume, and advise you accordingly. Additionally, sterilization tests can be performed with ethylene oxide and your product.
Below you will find more information about both sterilization processes.
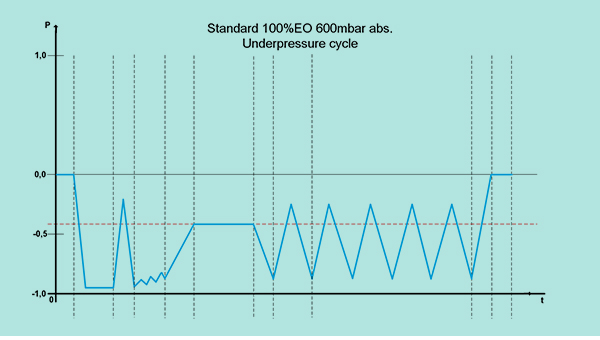
EO vacuum sterilization process
EO vacuum sterilization systems, among others, are offered by sterilization service providers, as this process is particularly well suited for medium to large production volumes. Medical devices are generally sterilized in their tertiary packaging on pallets. The latter are positioned in one or two rows in the chamber, oriented lengthwise or crosswise, depending on the customer and space requirements. STERISYS defines the exact chamber size together with you based on your production volume.
According to the current state of the art, the vacuum sterilization process consists of three phases:
- Preconditioning: preparation of medical devices for the sterilization process
- Sterilization: sterilization of devices in negative pressure
- Degassing: elimination of residual gas in the device according to EN ISO 10993-7
Standard EO gas concentrations for the EO vacuum sterilization process:
- 100% EO gas, packed in drums;
- 90% EO – 10% CO2.
As these EO concentrations are so high, the sterilization system must be designed and manufactured in accordance with the ATEX guidelines.
Through the use of 3D modulation, STERISYS also provides you with support in designing the entire sterilization system and the optimal production flow. The sterilization chamber (including all the necessary process components), the preconditioning chambers, the degassing chambers, the gas treatment unit and also the control room are integrated into your existing or new infrastructure.

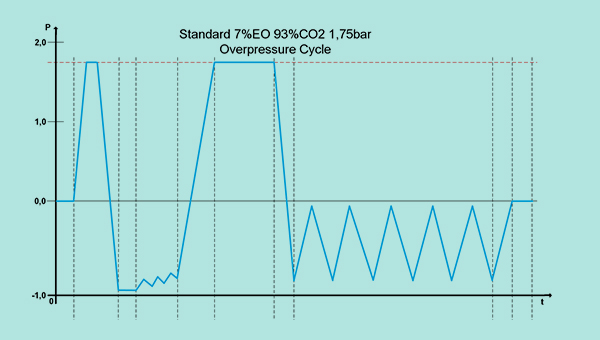
EO overpressure sterilization process
The EO overpressure process is particularly well suited for companies with small to medium production volumes. Here medical devices are mainly sterilized in their primary packaging, using a gas blend of, for example, 7% EO and 93% CO2. In contrast to the vacuum process, the gas holding phase during the sterilization cycle is held, for example, at an overpressure of 1.75 bar.
Dimensions of the overpressure sterilization system are determined based on your requirements. Considering the devices to be sterilized, we design suitable baskets, sterilization trolleys and the sterilization chamber together with you.
In addition, STERISYS helps you to seamlessly integrate the EO overpressure sterilizer into your new or existing processes.
Benefits of the overpressure process:
- Due to the low EO concentration, the ATEX guidelines are not applicable (conditions: max. EO gas concentration = 8% and/or LEL < 2.6%).
- The entire sterilization process – the preconditioning, sterilization and degassing of devices – can be carried out in the sterilization chamber. This provides space savings.
- The overall investment costs are lower for the EO overpressure process.
- In comparison to the EO vacuum process, cycle times are shorter, thus the devices reach the market more quickly.

EO dual sterilization process
Do you have both devices that are best sterilized in the overpressure process and also devices for which the vacuum sterilization process is more suitable? To eliminate the need to purchase two separate sterilization systems, STERISYS has developed an innovative EO dual system which allows you to operate both sterilization processes.
System features
Sterilization processes can be precisely parameterized, reproduced and documented.
Together with you, we analyze your requirements and advise you on the sterilization process, the appropriate system and the necessary subsystems.
Our sterilization systems and associated subsystems are always developed, manufactured and commissioned at your site in accordance with the newest standards and regulations.
Although gas treatment units are mandatory for both EO sterilization processes, other subsystems, for example, external preconditioning and degassing chambers, can be connected to the sterilization system for each customer specifically.




